

SIBIONICS
Innovating for You: Revolutionizing Diabetes Care with Every Step.
Since our founding in 2015, SIBIONICS has been at the forefront of transforming diabetes management. Born from a commitment to innovation and a profound understanding of the everyday challenges faced by those with diabetes, we've consistently aimed to redefine the landscape of Continuous Glucose Monitoring (CGM) systems. By merging cutting-edge technology with designs centered around the user, we deliver solutions that not only meet needs but exceed expectations.
Today, with over 1,000,000 users across the globe, SIBIONICS stands as a beacon of reliability and innovation in diabetes care. Our global presence is a testament to the effectiveness of our CGM solutions and our dedication to making a significant, positive impact worldwide.
Looking ahead, SIBIONICS is committed to continuing our legacy of innovation. We're not just keeping pace; we're setting the pace—constantly pushing the boundaries of what's possible in CGM technology and striving for excellence with each new product we introduce.
Innovera underliggande teknologier för att tjäna folkhälsan
Vi fokuserar på hälsovårdsbehoven hos den mest omfattande befolkningen och bryr oss också om sjukdomsdiagnostik och terapeutiska regimer för populationer med sällsynta sjukdomar. Vi har integrerat ett utmärkt forskarteam för att kontinuerligt omvandla banbrytande vetenskapliga forskningsresultat till medicinska produkter och tjäna folkhälsan.

Teknikens insikter i det okända väcker potential för liv
Baserat på patientbehov introducerar vi biokemi, chipdesign, artificiell intelligens, automation och andra teknologier för att få insikt i de okända områdena av hälsodata och hjälpa människor att göra förändringar mot en bättre livskvalitet
Certifikat
Vi är dedikerade till att hjälpa kunder att förbättra produktkvaliteten och ta itu med utmaningar.

Milstolpar och produktutbud

2023
The GS1 CGM system received the CE mark for entering the European market.

2021
The continuous glucose monitoring system (CGM) was submitted to National Medical Products Administration for registration and was approved. The practicing license of Yinchuan Sibionics Internet Hospital was approved.

2020
AI-DR was approved as the first Class III medical device for AI-assisted fundus disease diagnosis software in China. Sibionics was certified as National High-tech Enterprise.

2019
Fundus camera obtained the registration certificate for Class II medical device. R&D of capsule endoscopy project was started.
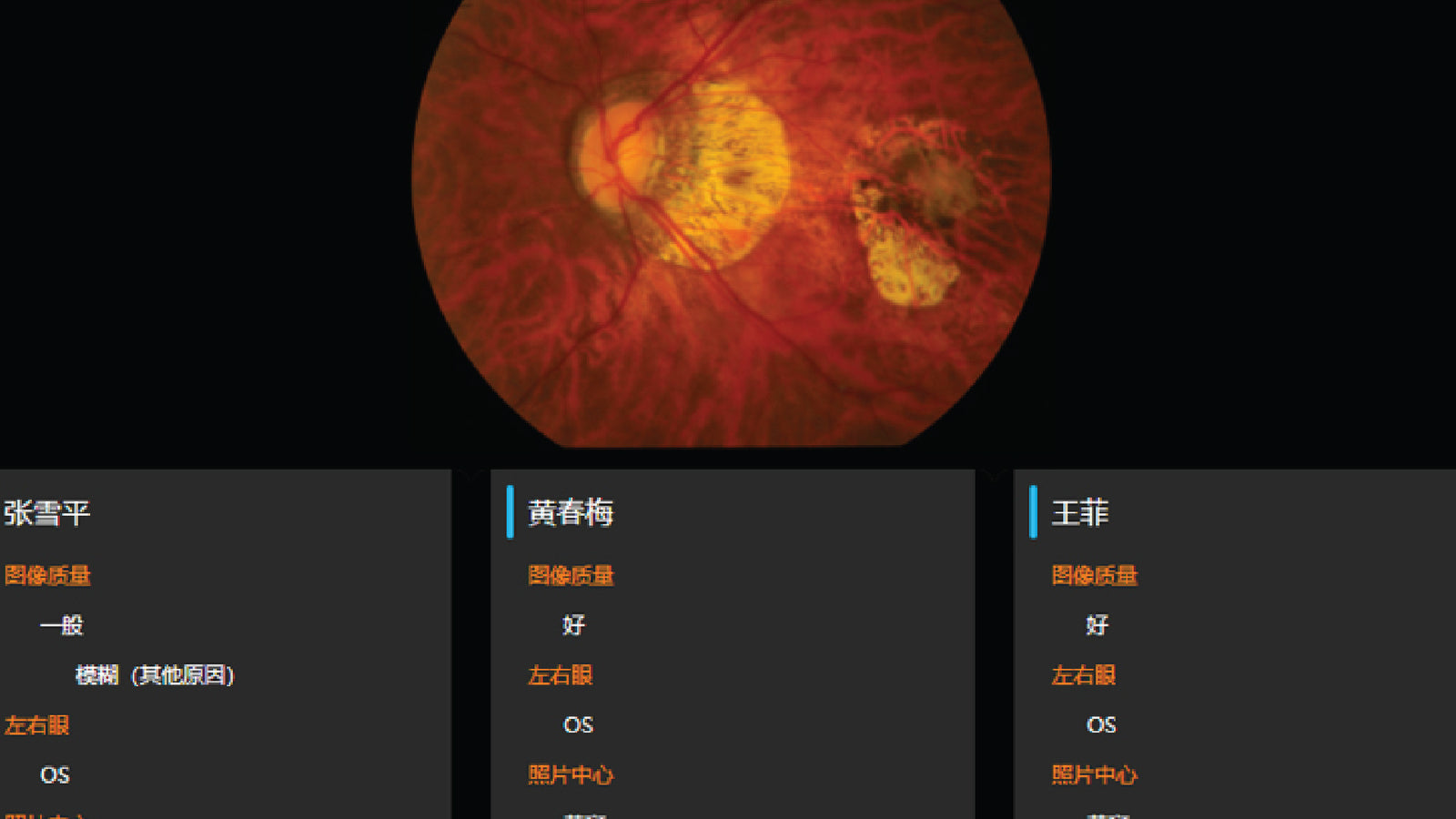
2016
R&D of continuous glucose monitoring system (CGM) was started. R&D of diabetic retinopathy AI-assisted diagnosis software (AI-DR) was started.
2015
Sibionics was established, and American R&D Center was established. R&D of artificial retina project was started.


