
SIBIONICS
Established in 2015, SIBIONICS was founded on the principles of innovation and a deep understanding of the challenges faced by individuals managing diabetes. From its inception, the brand set out to redefine the CGM system landscape by integrating state-of-the-art technology with user-centric design, delivering solutions that truly make a difference. With a user base exceeding 600,000 individuals worldwide, SIBIONICS has become a trusted companion in the daily lives of those navigating the complexities of diabetes management. Our global footprint reflects not only the efficacy of our CGM solutions but also the brand's commitment to making a positive impact on a global scale. In the future, SIBIONICS will continue to push the boundaries of CGM technology, striving for excellence with every new iteration.
Innovate underlying technologies to serve public health
We focus on the health management needs of the most extensive population, and also care about disease diagnosis and therapeutic regimens of populations with rare diseases. We have integrated an excellent scientist team to continuously transform cutting-edge scientific research achievements into medical products, and serve the public health.

Technology insights into the unknown arouse potential for life
Based on patient needs, we introduce biochemistry, chip design, artificial intelligence, automation and other technologies to gain insight into the unknown areas of health data and help people make changes towards a better quality of life
Certificates
We are dedicated to assisting clients to enhance product quality and address challenges.

Milestones and Product Lineup

2023
The GS1 CGM system received the CE mark for entering the European market.

2021
The continuous glucose monitoring system (CGM) was submitted to National Medical Products Administration for registration and was approved. The practicing license of Yinchuan Sibionics Internet Hospital was approved.

2020
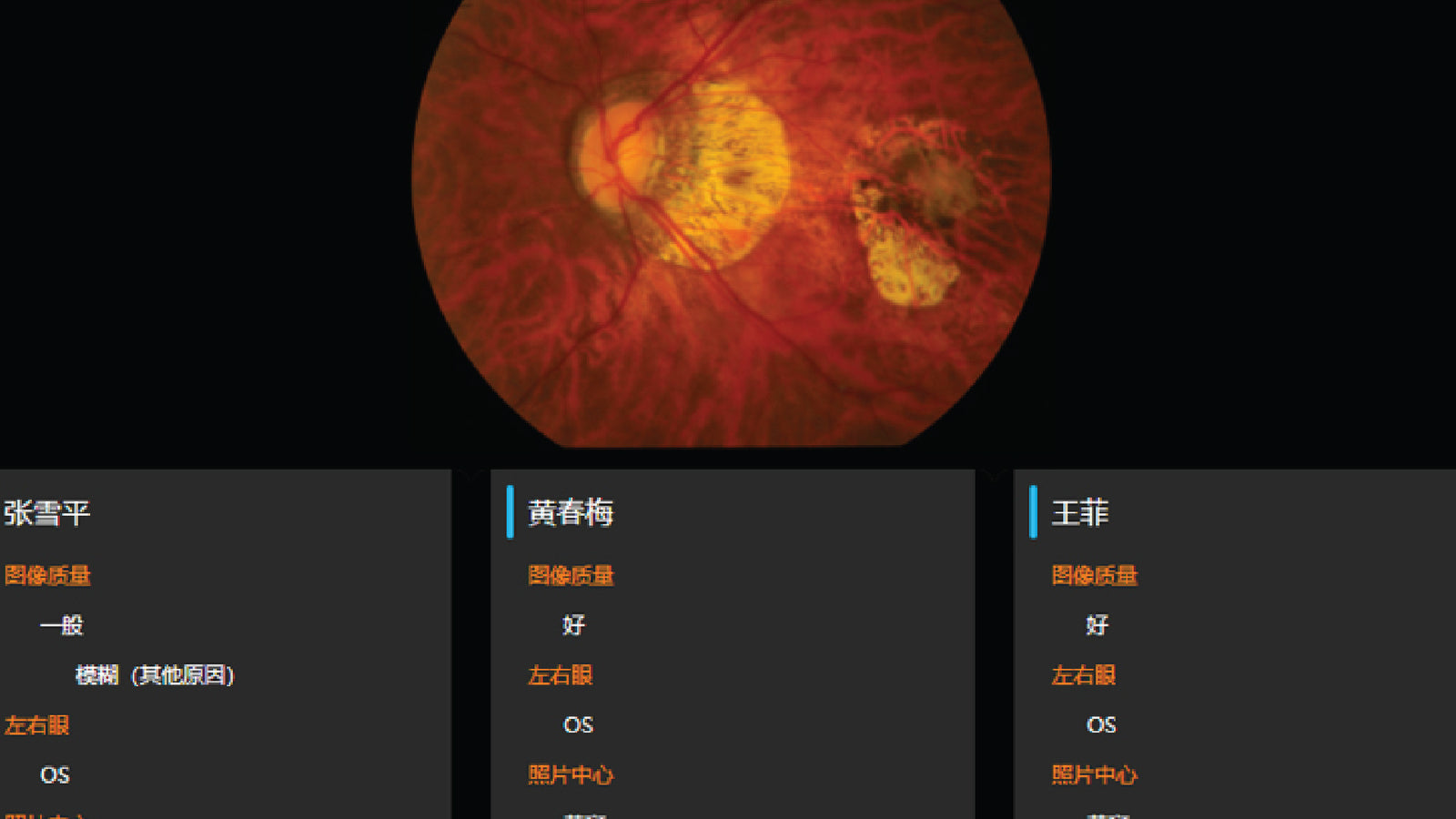
AI-DR was approved as the first Class III medical device for AI-assisted fundus disease diagnosis software in China. Sibionics was certified as National High-tech Enterprise.

2019
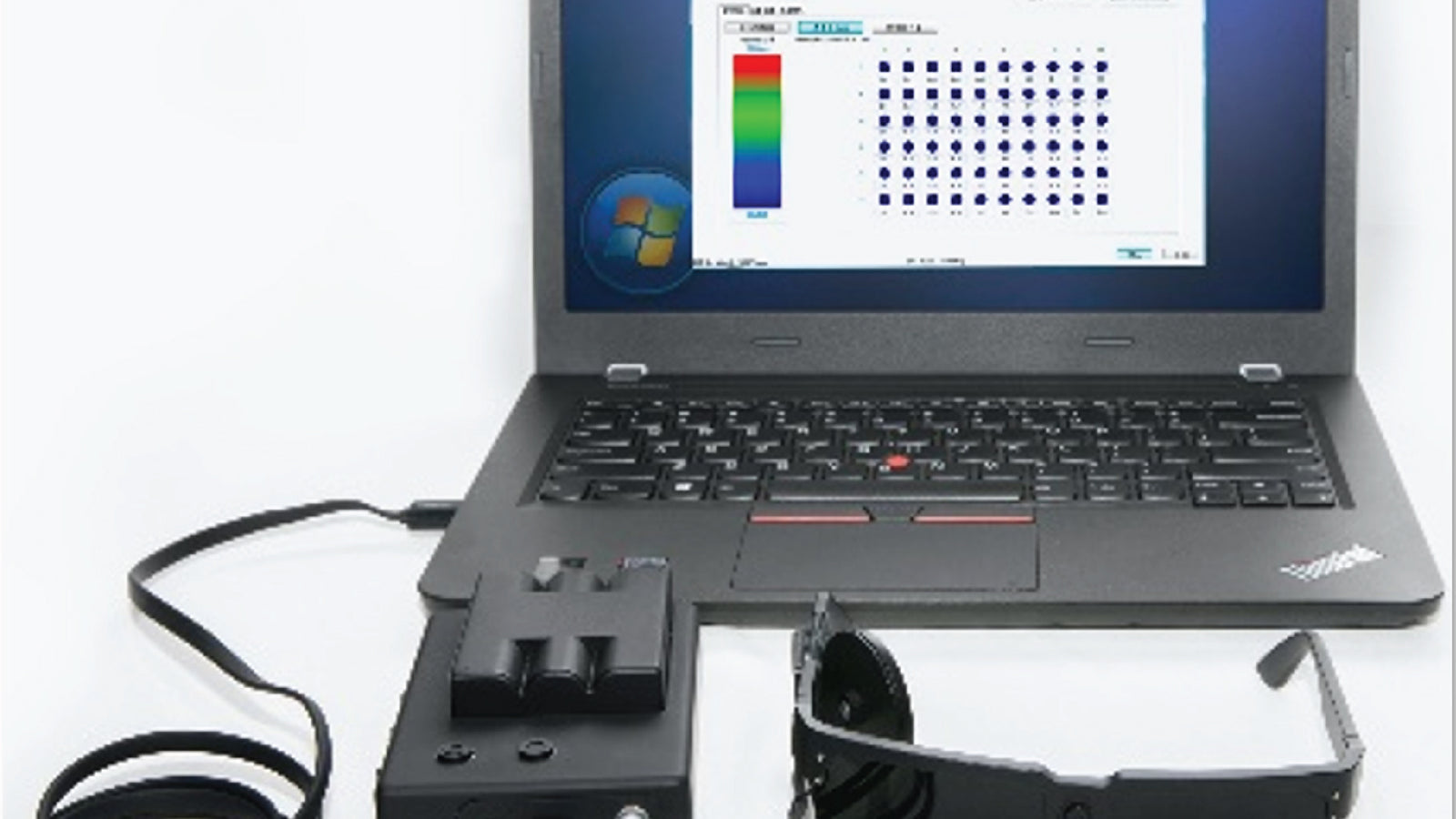
Fundus camera obtained the registration certificate for Class II medical device. R&D of capsule endoscopy project was started.
2016
R&D of continuous glucose monitoring system (CGM) was started. R&D of diabetic retinopathy AI-assisted diagnosis software (AI-DR) was started.
2015
Sibionics was established, and American R&D Center was established. R&D of artificial retina project was started.


